In 2012, a scientific discovery quietly set the stage for what might become one of the most transformative revolutions in human history. Researchers Jennifer Doudna and Emmanuelle Charpentier published a paper describing a bacterial defense mechanism that could be repurposed as a gene-editing tool. That tool was CRISPR-Cas9, and within just a few years, it became a household name in scientific and medical circles.
Today, CRISPR is at the center of bold new efforts to treat genetic diseases, engineer food, fight pandemics, and even alter entire ecosystems. As with any powerful technology, the possibilities are both exciting and ethically complex. Here’s a look at what CRISPR is, how it works, and what its future may hold.
What Is CRISPR?
CRISPR stands for Clustered Regularly Interspaced Short Palindromic Repeats—segments of DNA found in bacteria that help them defend against viruses. The CRISPR system works by storing snippets of viral DNA, which allows bacteria to recognize and cut up the same viruses if they attack again.
Scientists realized they could repurpose this system to cut and edit DNA in any organism, not just bacteria. The breakthrough came with CRISPR-Cas9, where Cas9 is a protein that acts like molecular scissors, guided by a piece of RNA that tells it exactly where to cut.
The beauty of CRISPR lies in its precision, speed, and affordability. What once took years and millions of dollars can now be done in weeks for a fraction of the cost.
How CRISPR Works
CRISPR-Cas9 uses two key components:
- A guide RNA (gRNA) designed to match a specific DNA sequence in the genome.
- The Cas9 enzyme, which cuts the DNA at the targeted location.
Once the DNA is cut, the cell’s natural repair machinery kicks in. Scientists can use this moment to:
- Disable a gene (by causing a disruptive mutation),
- Repair a gene (by supplying a correct copy), or
- Insert new genetic material (a process known as gene knock-in).
This technique gives scientists unprecedented control over genetic material in plants, animals, and humans.
Current Applications of CRISPR
1. Medicine and Disease Treatment
One of the most promising uses of CRISPR is in treating genetic disorders. Diseases like sickle cell anemia, cystic fibrosis, and muscular dystrophy are caused by specific gene mutations. With CRISPR, researchers can attempt to repair or disable the faulty genes directly.
In 2020, the first clinical trials using CRISPR to treat sickle cell disease and beta-thalassemia showed encouraging results, with patients producing healthy red blood cells after one treatment.
CRISPR is also being explored to fight:
- Cancer, by modifying immune cells to better attack tumors.
- HIV, by targeting and disrupting viral DNA integrated into host cells.
- Blindness, with trials underway to correct inherited retinal diseases.
2. Agriculture and Food Security
CRISPR is revolutionizing crop engineering. Scientists are using it to:
- Make plants more resistant to droughts, pests, and disease.
- Improve nutritional content, such as rice fortified with higher vitamin levels.
- Reduce reliance on chemical pesticides by breeding more resilient plants.
Unlike traditional GMOs, which often involve introducing foreign DNA, CRISPR can make precise changes within the plant’s own genome, which may help ease regulatory and consumer concerns.
3. Pandemics and Infectious Diseases
CRISPR is being used to develop diagnostics and treatments for viral diseases. During the COVID-19 pandemic, CRISPR-based tests were developed that could detect the virus quickly and accurately.
Researchers are also exploring “gene drives” using CRISPR to control mosquito populations that spread diseases like malaria or Zika. Gene drives ensure that a modified gene spreads quickly through a population, potentially wiping out disease-carrying insects.
4. Animal and Environmental Research
CRISPR is helping scientists create animal models for research more easily than ever before. These models are critical for understanding human disease and testing new treatments.
There’s also ongoing debate about de-extinction—using CRISPR to revive extinct species or restore traits in endangered ones, such as the woolly mammoth or passenger pigeon. While still theoretical, these projects raise questions about conservation and human responsibility.
The Ethical Landscape

The power of CRISPR brings serious ethical questions. The most controversial area is human germline editing—modifying embryos in ways that can be inherited by future generations. In 2018, a Chinese scientist announced he had edited the genes of twin babies to make them resistant to HIV. The backlash was immediate and global, with widespread condemnation from the scientific community.
Key ethical concerns include:
- Safety: Off-target edits could cause unintended harm.
- Consent: Future generations can’t consent to inherited genetic changes.
- Equity: Could CRISPR widen the gap between those who can afford enhancements and those who cannot?
- Eugenics: Where do we draw the line between therapy and enhancement?
Many scientists and policymakers are calling for a global framework to govern CRISPR’s use, especially in humans.
The Future of Gene Editing with CRISPR
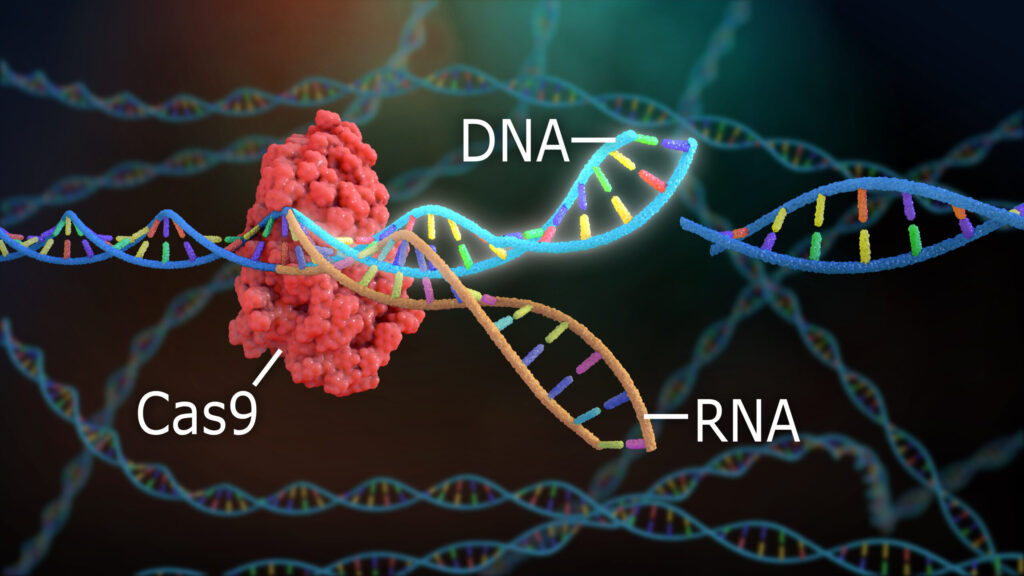
While CRISPR-Cas9 is revolutionary, it’s not perfect. Scientists are already developing next-generation tools to improve accuracy and reduce side effects.
Emerging Innovations:
- Base editing: Allows scientists to change individual DNA letters (A, T, C, G) without cutting the DNA strand—a gentler, more precise approach.
- Prime editing: Introduced in 2019, this tool can search and replace whole segments of DNA like a word processor, potentially fixing up to 89% of known genetic mutations.
- CRISPR-Cas12 and Cas13: These variants target RNA instead of DNA and are opening new frontiers in diagnostics and antiviral therapies.
Long-Term Possibilities:
- Curing all single-gene disorders, such as Huntington’s or Tay-Sachs.
- Eradicating certain cancers with personalized immune cell editing.
- Universal flu vaccines and treatments for viral outbreaks.
- Bioengineered organs for transplantation.
- Designer crops to feed a growing global population sustainably.
Conclusion
CRISPR is not just a scientific tool—it’s a transformative force that’s reshaping biology, medicine, agriculture, and even our ethical frameworks. We are only at the beginning of understanding what’s possible.
As gene editing becomes faster, cheaper, and more precise, the world must decide how to balance innovation with caution. Who gets access? What are the limits? How do we ensure it’s used for good?
The answers to these questions will define not just the future of science—but the future of humanity itself.